Dance of the Air Pollutants
Updated:
I wrote this blog for ClimaCell on how ozone and NO2 change within a day. Reposted from ‘ClimaCell Blog’
The most romantic part of the air pollution mechanism (i.e., atmospheric chemistry) is the dance between ozone (O3) and nitrogen dioxide (NO2) on the surface of Earth. During this late night air molecule tango one pollutant takes a step forward, the other takes a step back. Following the rhythm set by the sun, they take turns leading the dance. As the sun rises, ozone plays the leading role. When dusk falls, O3 yields to NO2.
We video-captured this dance in Los Angeles on October 29 2019, between midnight and 11:00 pm. The top two panels depict the concentrations of O3 and NO2 in parts per billion (ppb). Background colors are archives of real-time pollutant concentrations from ClimaCell, and the colored circles are the data from ‘AirNow’ (a governmental air pollution data platform).
Roughly, when O3 exceeds 70 ppb or NO2 exceeds 100 ppb, it means bad air quality – based on ‘National Ambient Air Quality Standards’. The lower panel is the diurnal cycle of the two pollutants. The x-axis ranges from 00:00 to 23:00 local time, and y-axis represents the pollutant concentrations in parts per billion.
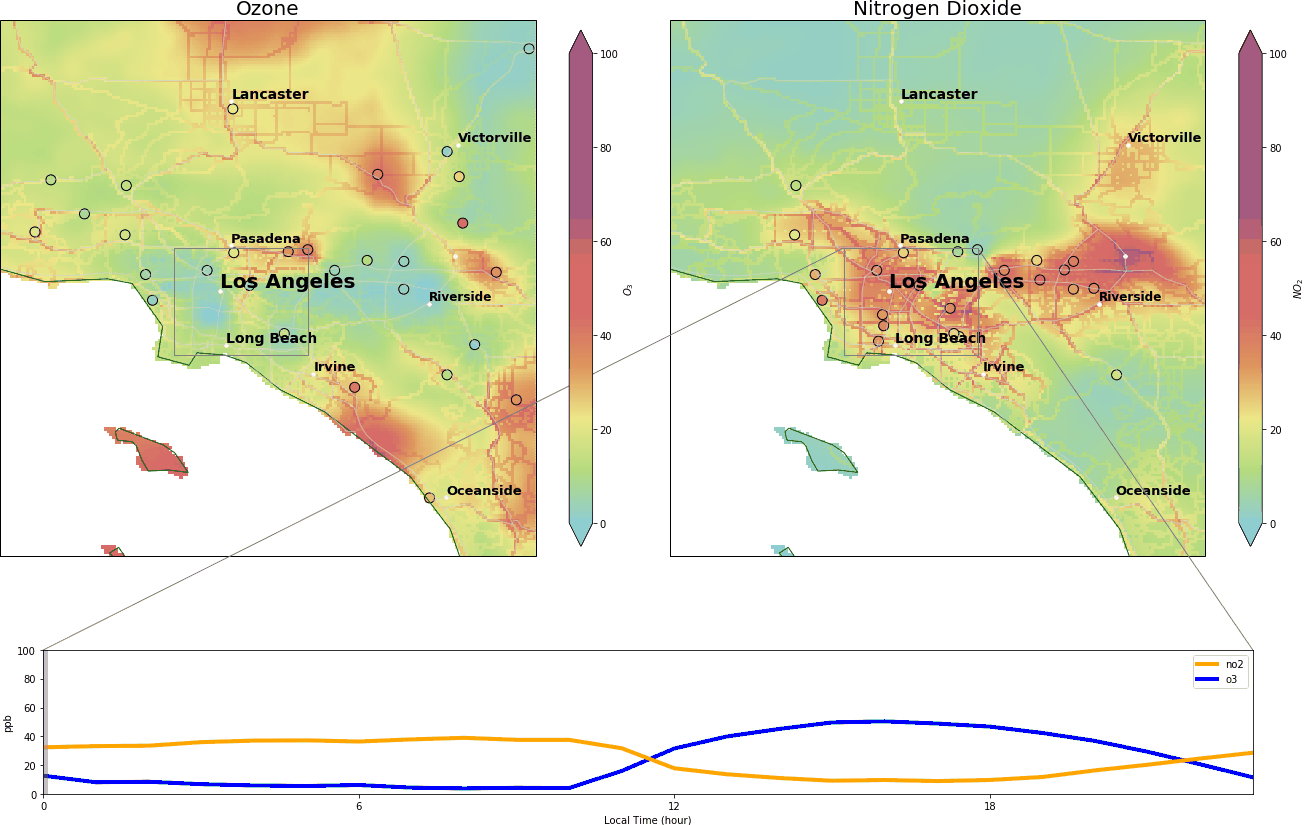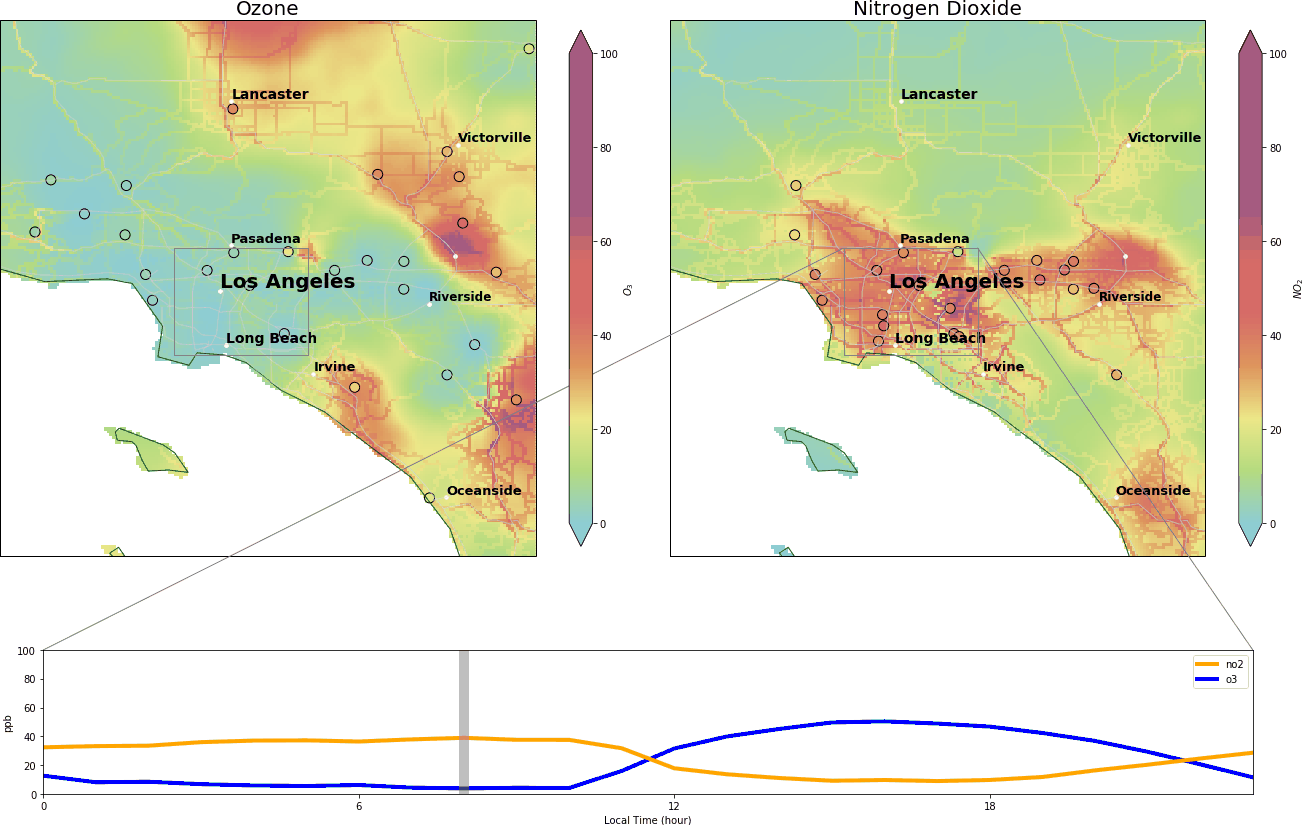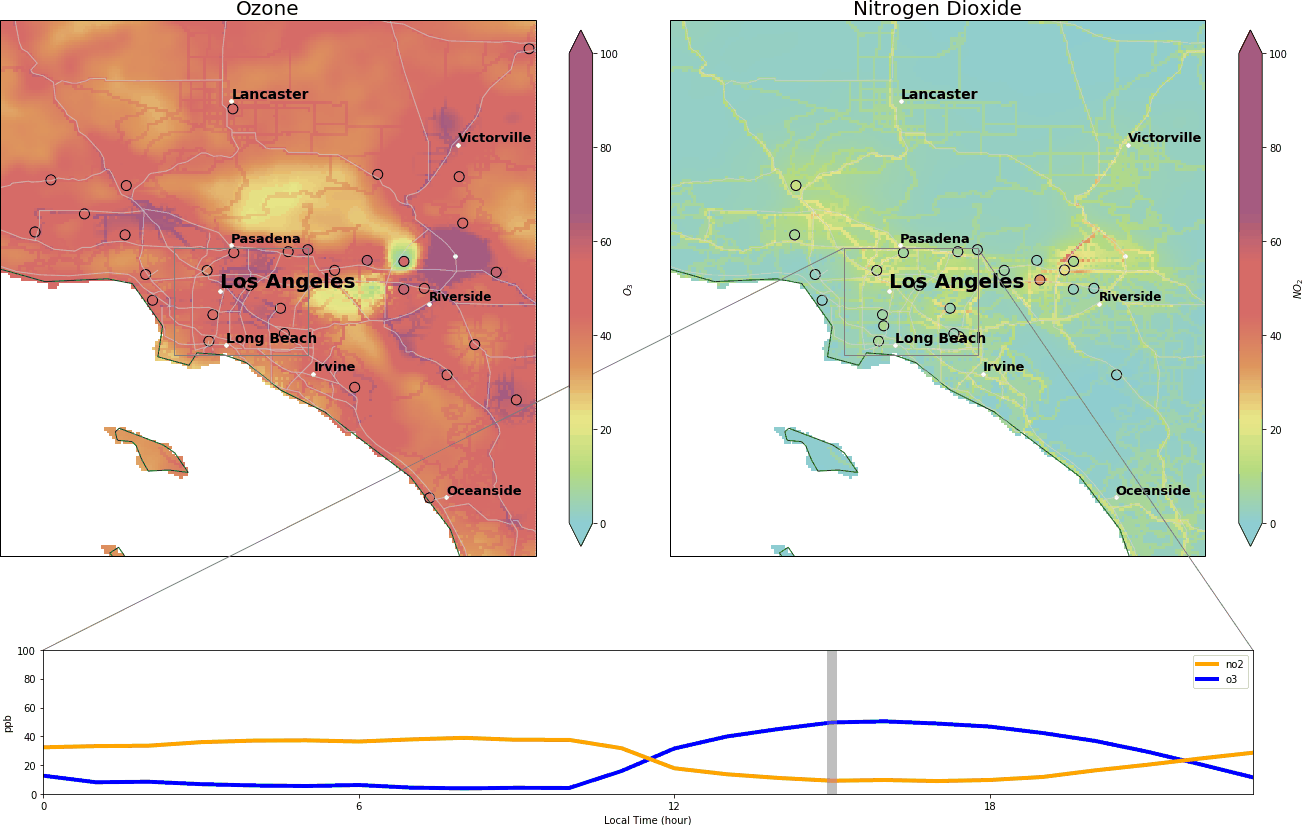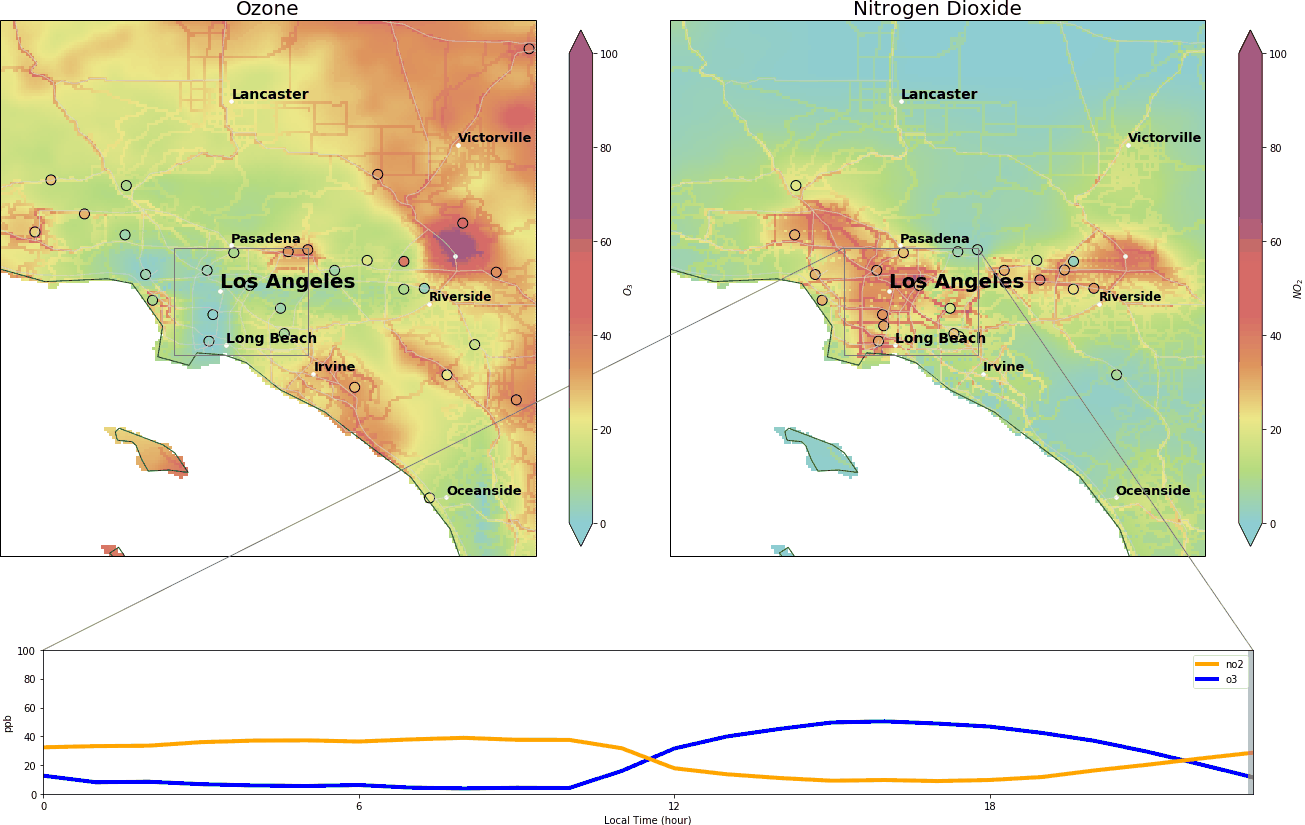
During the wee small hours, O3 gracefully drops to below 5 ppb and regains its spirit at around 9:00 am, peaking at over 40 ppb around 14:00. Meanwhile, NO2 drops after 9:00 am and reaches its nadir around 14:00.
Reads like a great partnership, doesn’t it?
So what’s the trick? Stratospheric O3 (good ozone protecting us from UV) high above us is produced from oxygen (O2) directly. But in the lower troposphere where we live, ozone is bad and can be produced by robbing one oxygen atom from NO2.
That’s why NO2 stays low during the daytime. The chemical reaction is:
O2 (oxygen) + NO2 + hv (sun light) → O3+ NO (nitrogen monoxide)
When the sunlight’s gone and night comes, O3 proves to be most generous and gives the oxygen atom back to NO2. Fresh NO is still produced during the nighttime, via fossil fuel combustion. NO grasps the oxygen, and sometimes leaves a little O3 behind. Hereby, we see very low levels of O3 during the nighttime.
The take-away formula is:
O3 + NO → O2 + NO2
One good turn deserves another…

Leave a comment